A systematic review/meta-analysis published in JAMA (Journal of the American Medical Association) in June 2015 evaluated the efficacy of cannabinoids in the treatment of various symptoms, diseases, and disorders.
Results of the Review
Using 28 databases to find and analyze 79 different trials with a total of 6,462 participants, the authors conducted the review on the use of cannabinoids for the following signs, symptoms, diseases/disorders:
- nausea and vomiting due to chemotherapy
- appetite stimulation in HIV/AIDS
- chronic pain
- spasticity due to multiple sclerosis or paraplegia
- depression
- anxiety disorder
- sleep disorder
- psychosis
- glaucoma
- Tourette syndrome
They found:
MODERATE-QUALITY evidence to support the use of cannabinoids for the treatment of:
LOW-QUALITY evidence to support the association between cannabinoid use and improvements in:
- nausea and vomiting due to chemotherapy
- weight gain in HIV infection
- sleep disorders
- Tourette syndrome
Additionally, participants using cannabinoid therapies were more likely to experience short-term and generally minimal adverse events including “dizziness, dry mouth, nausea, fatigue, somnolence, euphoria, vomiting, disorientation, drowsiness, confusion, loss of balance, and hallucination.”
Why Does It Matter That This Is a Systematic Review?
In the “hierarchy of evidence,”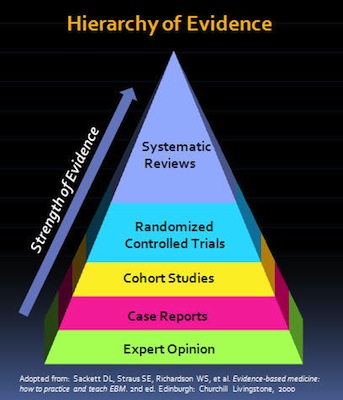 the systematic review is the type of study that uses, and is most likely to further, the highest quality evidence that can be gathered on a topic. A meta-analysis is like the quantitative (more objective) equivalent of the qualitative (more subjective) systematic review.
the systematic review is the type of study that uses, and is most likely to further, the highest quality evidence that can be gathered on a topic. A meta-analysis is like the quantitative (more objective) equivalent of the qualitative (more subjective) systematic review.
Since systematic reviews are conducted on multiple studies, they allow for a comprehensive and thorough review with less error overall. While individual chart studies or clinical trials also provide very useful data (and are the very foundation of systematic reviews), given various factors, they are more strongly influenced by bias, which is likely to produce inaccurate or misleading results.
Interpretation of the Cannabinoid Study
In another important step for the medical cannabis movement, this systematic review was published in JAMA, a prominent peer-reviewed medical journal frequently reviewed by physicians and medical scientists across the world.
Given (1) that millions of Americans suffer from chronic pain and spasticity, (2) the difficulties in diagnosis and treatment, and (3) the limited and often toxic treatment options available for patients, finding alternative therapies is essential. Additionally, the favorable safety profile of whole-plant medical cannabis makes safe experimentation by informed patients under medical supervision very low-risk. Therefore, “moderate” effects caused by a relatively safe treatment option should be researched in-depth.
Additionally, while the evidence available for the use of cannabinoid therapies for the other disorders noted above was classified as “low quality”, it is possible that in the cases of these disorders:
- a less-than-optimal or inconsistent cannabinoid ratio/concentration was used in the studies,
- cannabinoids may prove useful as add-on therapies rather than first-line treatment in these disorders, or
- at minimum, there is some evidence to support their medicinal use.
The systematic review is highly likely to also be inherently biased by the fact that research on cannabinoid medicine has been limited by ideological bias and legal status. However, as noted, it is the highest quality study that can be conducted given the information available.
Conclusion
Although the study found “low” and “moderate”, as opposed to “high”, quality evidence for the use of cannabinoids in the treatment of the medical conditions described, this is still a a significant finding . Given that the Schedule I classification of cannabis defines it as having “no medicinal value” and a “lack of safety under medical supervision”, this review provides high-quality evidence supporting the inaccuracy of this classification. This adds to a growing body of evidence that supports the medicinal efficacy of cannabinoids, and points to a need for increased research on various cannabinoid ratios, concentrations, and delivery methods for certain signs, symptoms, diseases, and disorders.
For information on reasonable expectations and safety in considering whole-plant medical cannabis use, as well as how you can advocate to move cannabis out of the Schedule I controlled substance classification in order to increase research on phytocannabinoids in the United States, click here.
Latest
Coronavirus Strikes Massachusetts Cannabis Company Employees
Reassessing the Essential: Cannabis in the Time of a Pandemic
5 Reasons To Try Aspen Valley CBG Flower (30% Off)
High Times Cannabis Cups Go Virtual In Wake Of Coronavirus Pandemic
Drug Enforcement Administration Proposes Plan To Expand Cannabis Research
Ghana Legalizes Cannabis For Medicinal And Industrial Uses
The cheapest legal weed in Canada: Discover these cannabis ‘value brands’
Cannabis and coronavirus: Here’s what you need to know
cannabis designs
The Best Of
WHO Rules CBD Should Not Be a Scheduled Drug

Dr Cristina Sanchez PhD video interview on medical marijuana and cancer

Biochemist Dennis Hill interview; Cannabis oil as a cure for cancer.

The unofficial World Record holder for cannabis smoking part 1





